InVivoMAb anti-mouse IFNγ
Product Details
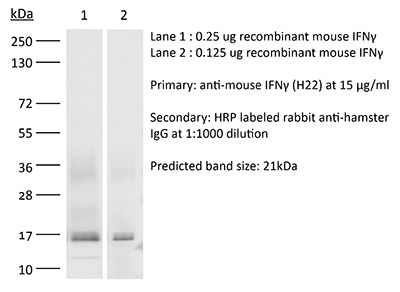
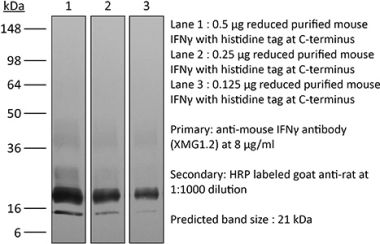
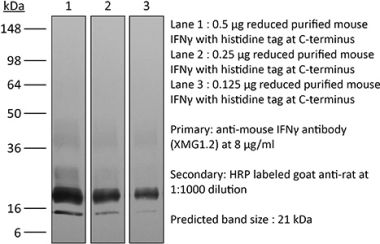
The H22 monoclonal antibody reacts with mouse IFNγ (interferon gamma) a 20 kDa soluble pleiotropic cytokine and the sole member of the type II class of interferons. IFNγ is primarily produced by activated lymphocytes including T, B, NK cells, and ILCs. IFNγ exerts immunoregulatory, anti-proliferative, anti-viral, and proinflammatory activities and plays an important role in activation, growth, and differentiation of T and B lymphocytes, macrophages, NK cells and other non-hematopoietic cell types. Additionally, IFNγ induces the production of cytokines, Fc receptor, and adhesion molecules and up-regulates MHC class I and II antigen expression by antigen presenting cells during an immune response. IFNγ has also been shown to modulate macrophage effector functions, influence isotype switching and induce the secretion of immunoglobulins by B cells. IFNγ signals through the IFN gamma receptor which exists as a heterodimer composed of CD119 (IFN gamma receptor 1) and AF-1 (IFN gamma receptor 2). The IFNγ receptor is expressed ubiquitously on almost all cell types with the exception of mature erythrocytes. The H22 antibody is a neutralizing antibody.Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant full-length murine IFNγ |
| Reported Applications |
in vivo IFNγ neutralization in vitro IFNγ neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2736992 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IFNγ neutralization
Slaney, C. Y., et al. (2017). "Dual-specific Chimeric Antigen Receptor T Cells and an Indirect Vaccine Eradicate a Variety of Large Solid Tumors in an Immunocompetent, Self-antigen Setting" Clin Cancer Res 23(10): 2478-2490. PubMed
Purpose: While adoptive transfer of T cells bearing a chimeric antigen receptor (CAR) can eliminate substantial burdens of some leukemias, the ultimate challenge remains the eradication of large solid tumors for most cancers. We aimed to develop an immunotherapy approach effective against large tumors in an immunocompetent, self-antigen preclinical mouse model.Experimental Design: In this study, we generated dual-specific T cells expressing both a CAR specific for Her2 and a TCR specific for the melanocyte protein (gp100). We used a regimen of adoptive cell transfer incorporating vaccination (ACTIV), with recombinant vaccinia virus expressing gp100, to treat a range of tumors including orthotopic breast tumors and large liver tumors.Results: ACTIV therapy induced durable complete remission of a variety of Her2(+) tumors, some in excess of 150 mm(2), in immunocompetent mice expressing Her2 in normal tissues, including the breast and brain. Vaccinia virus induced extensive proliferation of T cells, leading to massive infiltration of T cells into tumors. Durable tumor responses required the chemokine receptor CXCR3 and exogenous IL2, but were independent of IFNgamma. Mice were resistant to tumor rechallenge, indicating immune memory involving epitope spreading. Evidence of limited neurologic toxicity was observed, associated with infiltration of cerebellum by T cells, but was only transient.Conclusions: This study supports a view that it is possible to design a highly effective combination immunotherapy for solid cancers, with acceptable transient toxicity, even when the target antigen is also expressed in vital tissues. Clin Cancer Res; 23(10); 2478-90. (c)2016 AACR.
in vivo IFNγ neutralization
Nair, S., et al. (2017). "Interferon regulatory factor-1 (IRF-1) protects against chikungunya virus induced immunopathology by restricting infection in muscle cells" J Virol . PubMed
The innate immune system protects cells against viral pathogens in part through the autocrine and paracrine actions of interferons (IFN)-alpha/beta (type I), -gamma (type II), and -lambda (type III). The transcription factor interferon regulatory factor (IRF)-1 has a demonstrated role in shaping innate and adaptive antiviral immunity by inducing the expression of IFN stimulated genes (ISGs) and mediating signals downstream of IFN-gamma. Although ectopic expression experiments have suggested an inhibitory function of IRF-1 against infection of alphaviruses in cell culture, its role in vivo remains unknown. Here, we infected Irf1(-/-) mice with two distantly related arthritogenic alphaviruses, chikungunya (CHIKV) and Ross River (RRV), and assessed the early antiviral functions of IRF-1 prior to induction of adaptive B and T cell responses. IRF-1 expression limited CHIKV-induced foot swelling in joint-associated tissues and prevented dissemination of CHIKV and RRV at early time points. Virological and histological analysis revealed greater infection of muscle tissues in Irf1(-/-) compared to wild-type mice. The antiviral actions of IRF-1 appeared independent of the induction of type I IFN or effects of type II and III IFNs but were associated with altered local pro-inflammatory cytokine and chemokine responses and differential infiltration of myeloid cell subsets. Collectively, our in vivo experiments suggest that IRF-1 restricts CHIKV and RRV infection in stromal cells, especially muscle cells, and this controls local inflammation and joint-associated swelling.IMPORTANCE Interferon regulatory factor (IRF)-1 is a transcription factor that regulates the expression of a broad range of antiviral host defense genes. In this study, using Irf1(-/-) mice, we investigated the role of IRF-1 in modulating pathogenesis of two related arthritogenic alphaviruses, chikungunya and Ross River viruses. Our studies show that IRF-1 controlled alphavirus replication and swelling in joint-associated tissues within days of infection. Detailed histopathological and virological analyses revealed that IRF-1 preferentially restricted CHIKV infection in cells of non-hematopoietic lineage, including muscle cells. The antiviral actions of IRF-1 resulted in decreased local inflammatory responses in joint associated tissues, which prevented immunopathology.
in vivo IFNγ neutralization
Noguchi, T., et al. (2017). "Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape" Cancer Immunol Res 5(2): 106-117. PubMed
Antibody blockade of programmed death-1 (PD-1) or its ligand, PD-L1, has led to unprecedented therapeutic responses in certain tumor-bearing individuals, but PD-L1 expression’s prognostic value in stratifying cancer patients for such treatment remains unclear. Reports conflict on the significance of correlations between PD-L1 on tumor cells and positive clinical outcomes to PD-1/PD-L1 blockade. We investigated this issue using genomically related, clonal subsets from the same methylcholanthrene-induced sarcoma: a highly immunogenic subset that is spontaneously eliminated in vivo by adaptive immunity and a less immunogenic subset that forms tumors in immunocompetent mice, but is sensitive to PD-1/PD-L1 blockade therapy. Using CRISPR/Cas9-induced loss-of-function approaches and overexpression gain-of-function techniques, we confirmed that PD-L1 on tumor cells is key to promoting tumor escape. In addition, the capacity of PD-L1 to suppress antitumor responses was inversely proportional to tumor cell antigenicity. PD-L1 expression on host cells, particularly tumor-associated macrophages (TAM), was also important for tumor immune escape. We demonstrated that induction of PD-L1 on tumor cells was IFNgamma-dependent and transient, but PD-L1 induction on TAMs was of greater magnitude, only partially IFNgamma dependent, and was stable over time. Thus, PD-L1 expression on either tumor cells or host immune cells could lead to tumor escape from immune control, indicating that total PD-L1 expression in the immediate tumor microenvironment may represent a more accurate biomarker for predicting response to PD-1/PD-L1 blockade therapy, compared with monitoring PD-L1 expression on tumor cells alone. Cancer Immunol Res; 5(2); 106-17. (c)2017 AACR.
in vivo IFNγ neutralization
Wu, L. L., et al. (2014). "Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-gamma" Am J Pathol 184(8): 2260-2274. PubMed
Abnormal bacterial adherence and internalization in enterocytes have been documented in Crohn disease, celiac disease, surgical stress, and intestinal obstruction and are associated with low-level interferon (IFN)-gamma production. How commensals gain access to epithelial soma through densely packed microvilli rooted on the terminal web (TW) remains unclear. We investigated molecular and ultrastructural mechanisms of bacterial endocytosis, focusing on regulatory roles of IFN-gamma and myosin light chain kinase (MLCK) in TW myosin phosphorylation and brush border fanning. Mouse intestines were sham operated on or obstructed for 6 hours by loop ligation with intraluminally administered ML-7 (a MLCK inhibitor) or Y27632 (a Rho-associated kinase inhibitor). After intestinal obstruction, epithelial endocytosis and extraintestinal translocation of bacteria were observed in the absence of tight junctional damage. Enhanced TW myosin light chain phosphorylation, arc formation, and brush border fanning coincided with intermicrovillous bacterial penetration, which were inhibited by ML-7 and neutralizing anti-IFN-gamma but not Y27632. The phenomena were not seen in mice genetically deficient for long MLCK-210 or IFN-gamma. Stimulation of human Caco-2BBe cells with IFN-gamma caused MLCK-dependent TW arc formation and brush border fanning, which preceded caveolin-mediated bacterial internalization through cholesterol-rich lipid rafts. In conclusion, epithelial MLCK-activated brush border fanning by IFN-gamma promotes adherence and internalization of normally noninvasive enteric bacteria. Transcytotic commensal penetration may contribute to initiation or relapse of chronic inflammation.
in vivo IFNγ neutralization, in vitro IFNγ neutralization
Steed, A. L., et al. (2006). "Gamma interferon blocks gammaherpesvirus reactivation from latency" J Virol 80(1): 192-200. PubMed
Establishment of latent infection and reactivation from latency are critical aspects of herpesvirus infection and pathogenesis. Interfering with either of these steps in the herpesvirus life cycle may offer a novel strategy for controlling herpesvirus infection and associated disease pathogenesis. Prior studies show that mice deficient in gamma interferon (IFN-gamma) or the IFN-gamma receptor have elevated numbers of cells reactivating from murine gammaherpesvirus 68 (gammaHV68) latency, produce infectious virus after the establishment of latency, and develop large-vessel vasculitis. Here, we demonstrate that IFN-gamma is a powerful inhibitor of reactivation of gammaHV68 from latency in tissue culture. In vivo, IFN-gamma controls viral gene expression during latency. Importantly, depletion of IFN-gamma in latently infected mice results in an increased frequency of cells reactivating virus. This demonstrates that IFN-gamma is important for immune surveillance that limits reactivation of gammaHV68 from latency.
in vivo IFNγ neutralization
Leiby, D. A., et al. (1992). "In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies" Infect Immun 60(1): 84-89. PubMed
The role(s) of gamma interferon (IFN-gamma), tumor necrosis factor alpha (TNF-alpha), and interleukin-4 (IL-4) in establishment and maintenance of protective immunity to Francisella tularensis LVS in mice (C3H/HeN) was examined by selective removal of these cytokines in vivo with neutralizing antibodies. The 50% lethal dose (LD50) for mice infected intradermally with F. tularensis alone was 136,000 CFU; treatment of mice with anti-IFN-gamma or anti-TNF-alpha at the time of infection significantly reduced (P much less than 0.05) the LD50 to 2 and 5 CFU, respectively. Abrogation of protective immunity, however, was effective only when anti-IFN-gamma or anti-TNF-alpha was administered prior to day 3 postinfection. In contrast, the LD50 for mice treated with anti-IL-4 was repeatedly higher (555,000 CFU) than for controls; this difference, however, was not significant (P greater than 0.05). Thus, IL-4 may be detrimental, while IFN-gamma and TNF-alpha were clearly crucial to the establishment of protective immunity to F. tularensis during a primary infection. The importance of IFN-gamma and TNF-alpha during a secondary immune response to F. tularensis was also investigated. Spleen cells from immune mice passively transfer protective immunity to recipient mice in the absence of confounding antibody-mediated immunity. This passive transfer of immunity, however, was abrogated by treatment of recipient mice with anti-IFN-gamma or anti-TNF-alpha at the time of challenge infection. That anticytokines effectively abrogate protective immunity very early in the course of infection with F. tularensis suggests that T-cell-dependent activation of macrophages for microbicidal activity is unlikely. These T-cell-independent events early in the course of infection may suppress bacterial replication until a T-cell-dependent response ultimately clears the bacteria.
in vitro IFNγ neutralization
Schreiber, R. D., et al. (1985). "Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity" J Immunol 134(3): 1609-1618. PubMed
Four monoclonal IgG antibodies to purified, recombinant murine gamma-interferon (rIFN-gamma) have been produced by fusion of immune hamster splenocytes with HAT-sensitive murine myeloma cells. Specificity was confirmed either with an enzyme-linked immunosorbent assay (ELISA) that used immobilized rIFN-gamma or with a radioimmunoassay that employed soluble 125I-rIFN-gamma and heat-killed, fixed Staphylococcus aureus-bearing Protein A. Competition binding experiments suggested that the monoclonal antibodies (MoAb) displayed two distinct epitope specificities: one displayed by H1 and H2, and the other displayed by H21 and H22. By using murine-human recombinant IFN-gamma hybrid molecules, the H1/H2 epitope was shown to depend on the amino-terminus of IFN-gamma, whereas the H21/H22 epitope was formed by the carboxy-terminal amino acid sequence. The MoAb also reacted with natural IFN-gamma. When bound to a surface, all four MoAb, but not normal hamster IgG, removed 100% of the antiviral and MAF activities present in supernatants of cultures of the murine 24/G1 T cell hybridoma. In free solution, all four antibodies inhibited IFN-gamma dependent antiviral activity, but with different efficiencies. Soluble H21/H22 also blocked all of the 24/G1-derived activity that induces nonspecific tumoricidal activity in macrophages (MAF) while H1/H2 enhanced MAF activity. The differential inhibitory or enhancing activities of H21 or H1 reflected their ability to inhibit or enhance binding of 125I-rIFN-gamma to macrophages, respectively. Soluble H21/H22 and solid-phase H1/H2 inhibited 100% of the MAF, microbicidal, and Ia-inducing activities from lymphokine preparations produced by mitogen stimulation of normal murine splenic cells. These results help to establish definitive structure-function relationships for the IFN-gamma molecule, and indicate that IFN-gamma is the primary lymphokine responsible for inducing nonspecific tumoricidal activity and Ia antigen expression, and for enhancing microbicidal activity in macrophages.