InVivoMAb anti-mouse CD20
Product Details
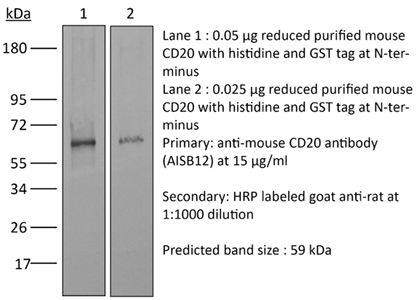
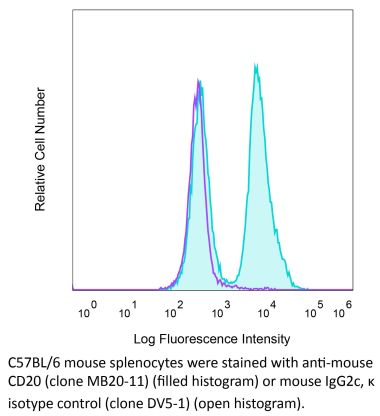
The AISB12 monoclonal antibody reacts with mouse CD20. CD20 is a B cell-specific 33-37 kDa transmembrane protein which is also known as B-lymphocyte antigen, B1, and Bp35. CD20 plays roles in intracellular calcium regulation and B cell activation and is critical for an optimal B cell immune response against T-independent antigens. CD20 is first expressed after the induction of CD19 together with IgM during the pre-B to immature B cell transition in the bone marrow. It’s expression then increases during maturation with almost all mature B cells expressing some level of CD20. However, CD20 is not expressed by plasma blasts or plasma cells. CD20 is expressed by most B cell neoplasms, and is useful in diagnosing B cell lymphomas and leukemia. Many anti-CD20 monoclonal antibodies are currently being used to successfully treat leukemia, lymphomas, and various autoimmune diseases.*Please note that the AISB12 clone is not suitable for in vivo B cell depletion. This antibody has little to no B cell depleting activity.
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Full length mouse CD20 protein |
| Reported Applications |
Flow cytometry Western blot Not recommended for in vivo B cell depletion |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2715460 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Flow Cytometry
Mishima, Y., et al. (2010). "Decreased production of interleukin-10 and transforming growth factor-beta in Toll-like receptor-activated intestinal B cells in SAMP1/Yit mice" Immunology 131(4): 473-487. PubMed
A unique subset of B cells expressing interleukin-10 (IL-10) and transforming growth factor-beta (TGF-beta) plays an essential role in preventing inflammation and autoimmunity. We investigated the presence of this cell subset in intestines and its role in the pathogenesis of ileitis using SAMP1/Yit and age-matched control AKR/J mice. Mononuclear cells were isolated from mesenteric lymph nodes (MLNs) and the expressions of B220, CD1d, CD5, Toll-like receptor 4 (TLR4) and TLR9 in isolated cells were analysed. Purified B cells were stimulated with lipopolysaccharide (LPS) or CpG-DNA, then IL-10 and TGF-beta(1) expressions were examined by enzyme immunoassay and flow cytometry. Production of IL-1beta by TLR-mediated macrophages co-cultured with or without purified MLN B cells from SAMP1/Yit and AKR/J mice was evaluated. In addition, interferon-gamma (IFN-gamma) production in intestinal T cells co-cultured with MLN B cells were also assessed in SAMP1/Yit and AKR/J strains. The production levels of IL-10 and TGF-beta(1) stimulated by LPS and CpG-DNA were significantly lower in B cells separated from MLNs from the SAMP1/Yit strain. B cells expressing IL-10 and TGF-beta(1) were mainly located in a population characterized by the cell surface marker CD1d(+) . Interleukin-1beta production by TLR-activated macrophages co-cultured with MLN B cells from SAMP1/Yit mice was significantly higher than that of those from AKR/J mice. Interestingly, IFN-gamma production by T cells was noted only when they were co-cultured with SAMP1/Yit but not the AKR/J B cells. These results are the first to show that disorders of regulatory B-cell function under innate immune activation may cause disease pathogenesis in a murine model of Crohn’s disease.