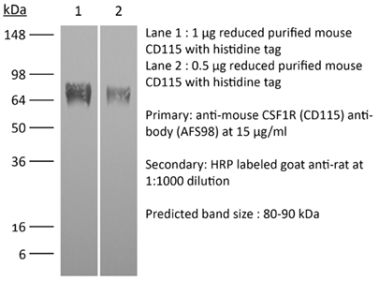
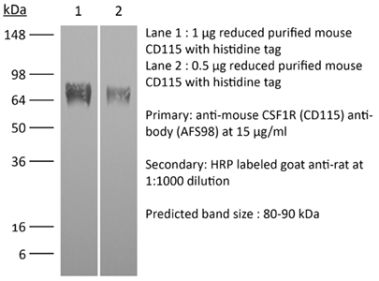
InVivoMAb anti-human CSF1R (CD115)
Product Details
The 2-4A5-4 monoclonal antibody reacts with human colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), CD115, and c-fms. CSF1R is a single-pass type I membrane protein and member of the platelet-derived growth factor receptor family. CSF1R is expressed by monocytes/macrophages, peritoneal exudate cells, plasmacytoid and conventional dendritic cells, and osteoclasts. CSF1R is a receptor for CSF1 and CSF1. Signaling through CSF1R regulates the proliferation and differentiation of cells in the monocytic lineage.Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Rat NRK cells expressing transduced human CSF1R |
| Reported Applications |
in vitro CSF1R neutralization Immunohistochemistry (paraffin) Functional assays Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2894766 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Immunohistochemistry (paraffin)
Hoffmann, S., et al. (2014). "Enlarging the nosological spectrum of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS)" Brain Pathol 24(5): 452-458. PubMed
A, p.R777Q) in the CSF1R gene. Through this report, we aim to enlarge the nosological spectrum of HDLS, providing new clinical descriptions as well as novel neuropathological findings from the peripheral nervous system.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) is an autosomal dominant disease clinically characterized by cognitive decline, personality changes, motor impairment, parkinsonism and seizures. Recently, mutations in the colony-stimulating factor-1 receptor (CSF1R) gene have been shown to be associated with HDLS. We report clinical, neuropathological and molecular genetic findings of patients from a new family with a mutation in the CSF1R gene. Disease onset was earlier and disease progression was more rapid compared with previously reported patients. Psychiatric symptoms including personality changes, alcohol abuse and severe depression were the first symptoms in male patients. In the index, female patient, the initial symptom was cognitive decline. Magnetic resonance imaging (MRI) showed bilateral, confluent white matter lesions in the cerebrum. Stereotactic biopsy revealed loss of myelin and microglial activation as well as macrophage infiltration of the parenchyma. Numerous axonal swellings and spheroids were present. Ultrastructural analysis revealed pigment-containing macrophages. Axonal swellings were detected by electron microscopy not only in the central nervous system (CNS) but also in skin nerves. We identified a heterozygous mutation (c.2330G>A, p.R777Q) in the CSF1R gene. Through this report, we aim to enlarge the nosological spectrum of HDLS, providing new clinical descriptions as well as novel neuropathological findings from the peripheral nervous system.
in vitro CSF1R neutralization, Functional Assays
Chihara, T., et al. (2010). "IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation" Cell Death Differ 17(12): 1917-1927. PubMed
Macrophage colony-stimulating factor (M-CSF) regulates the production, survival and function of macrophages through Fms, the receptor tyrosine kinase. Recently, interleukin-34 (IL-34), which shares no sequence homology with M-CSF, was identified as an alternative Fms ligand. Here, we provide the first evidence that these ligands indeed resemble but are not necessarily identical in biological activity and signal activation. In culture systems tested, IL-34 and M-CSF showed an equivalent ability to support cell growth or survival. However, they were different in the ability to induce the production of chemokines such as MCP-1 and eotaxin-2 in primary macrophages, the morphological change in TF-1-fms cells and the migration of J774A.1 cells. Importantly, IL-34 induced a stronger but transient tyrosine phosphorylation of Fms and downstream molecules, and rapidly downregulated Fms. Even in the comparison of active domains, these ligands showed no sequence homology including the position of cysteines. Interestingly, an anti-Fms monoclonal antibody (Mab) blocked both IL-34-Fms and M-CSF-Fms binding, but another MAb blocked only M-CSF-Fms binding. These results suggested that IL-34 and M-CSF differed in their structure and Fms domains that they bound, which caused different bioactivities and signal activation kinetics/strength. Our findings indicate that macrophage phenotype and function are differentially regulated even at the level of the single receptor, Fms.
in vitro CSF1R neutralization
Fuhrman, B., et al. (2008). "Ox-LDL induces monocyte-to-macrophage differentiation in vivo: Possible role for the macrophage colony stimulating factor receptor (M-CSF-R)" Atherosclerosis 196(2): 598-607. PubMed
Monocyte-to-macrophage differentiation and LDL oxidation play a pivotal role in early atherogenesis. We thus questioned possible mechanisms for oxidized LDL (Ox-LDL)-induced monocyte-to-macrophage differentiation in vivo. Mouse peritoneal mononuclear cells, that were isolated 1, 2, or 3 days after Ox-LDL intraperitoneal injection, gradually exhibited the characteristic macrophage morphology, along with the expression of the cell-surface antigen CD11b. Molecular mechanisms involved in Ox-LDL-induced differentiation were further investigated in vitro using the THP-1 monocytic cell line. THP-1 cells incubated with Ox-LDL in the presence of as low as 1 ng/ml of PMA differentiated into macrophages, as evidenced by morphologic, phenotypic, and functional properties. Stimulation of monocyte-to-macrophage differentiation was selective to Ox-LDL (and not native LDL), was dependent on the extent of LDL oxidation, and required Ox-LDL internalization by the cells. These effects of Ox-LDL could be attributed to its major oxysterols, 7-ketocholesterol and 7beta-hydroxycholesterol. Finally, the stimulation of monocyte differentiation to macrophages by Ox-LDL was shown to require the M-CSF-receptor, since blocking the binding to the receptor abolished Ox-LDL/7beta-hydroxycholesterol-induced differentiation. Furthermore, Ox-LDL/7beta-hydroxycholesterol elicited tyrosine phosphorylation and activation of the M-CSF-R. We thus conclude that Ox-LDL induces monocyte differentiation to macrophages in vivo and this phenomenon involves activation of the M-CSF-receptor.
in vitro CSF1R neutralization
Kirma, N., et al. (2007). "Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-beta1 in inducing c-fms expression" Cancer Res 67(5): 1918-1926. PubMed
Cervical cancer is the third most common gynecologic cancer in the United States. The presence and possible involvement of several cytokines have been studied in cervical cancer; however, very little data, if any, are available on whether cervical tumors are responsive to stimulation by the macrophage colony-stimulating factor-1 (CSF-1). Given the involvement of c-fms and its ligand CSF-1 in gynecologic cancers, such as that of the uterus and the ovaries, we have examined the expression of c-fms and CSF-1 in cervical tumor (n = 17) and normal cervix (n = 8) samples. The data show that c-fms and its ligand are significantly higher in cervical carcinomas compared with normal samples. Immunohistochemistry not only showed that tumor cells expressed significantly higher levels of c-fms but also c-fms levels were markedly higher in tumor cells than tumor-associated stromal cells. Blocking c-fms activity in cervical cancer cells, which express CSF-1 and c-fms, resulted in increased apoptosis and decreased motility compared with control, suggesting that CSF-1/c-fms signaling may be involved in enhanced survival and possibly invasion by cervical cancer cells via an autocrine mechanism. Combined, the data show for the first time the induction of CSF-1 and c-fms in cervical carcinomas and suggest that c-fms activation may play a role in cervical carcinogenesis. Additionally, our data suggest that transforming growth factor-beta1 may be a factor in inducing the expression of c-fms in cervical cancer cells. The data suggest that c-fms may be a valuable therapeutic target in cervical cancer.
Flow Cytometry
Tan, B. L., et al. (2003). "Functional and biochemical consequences of abrogating the activation of multiple diverse early signaling pathways in Kit. Role for Src kinase pathway in Kit-induced cooperation with erythropoietin receptor" J Biol Chem 278(13): 11686-11695. PubMed
Kit receptor tyrosine kinase and erythropoietin receptor (Epo-R) cooperate in regulating blood cell development. Mice that lack the expression of Kit or Epo-R die in utero of severe anemia. Stimulation of Kit by its ligand, stem cell factor activates several distinct early signaling pathways, including phospholipase C gamma, phosphatidylinositol 3-kinase, Src kinase, Grb2, and Grb7. The role of these pathways in Kit-induced growth, proliferation, or cooperation with Epo-R is not known. We demonstrate that inactivation of any one of these early signaling pathways in Kit significantly impairs growth and proliferation. However, inactivation of the Src pathway demonstrated the most profound defect. Combined stimulation with Epo also resulted in impaired cooperation between Src-defective Kit mutant and Epo-R and, to a lesser extent, with Kit mutants defective in the activation of phosphatidylinositol 3-kinase or Grb2. The impaired cooperation between the Src-defective Kit mutant and Epo-R was associated with reduced transphosphorylation of Epo-R and expression of c-Myc. Remarkably, restoration of only the Src pathway in a Kit receptor defective in the activation of all early signaling pathways demonstrated a 50% correction in proliferation in response to Kit stimulation and completely restored the cooperation with Epo-R. These data demonstrate an essential role for Src pathway in regulating growth, proliferation, and cooperation with Epo-R downstream from Kit.
in vitro CSF1R neutralization
Rosenfeld, C. S.. (1994). "Transforming growth factor-beta 1 augments macrophage-colony stimulating factor activity on human marrow" Stem Cells 12(5): 527-532. PubMed
Transforming growth factor-beta 1 (TGF-beta 1) suppresses the colony stimulating activity of most cytokines. The effect of TGF-beta 1 on macrophage colonies induced by macrophage colony stimulating factor (M-CSF) from human marrow has not been described. Experiments were performed with phenylalanine methyl ester (PME) treated marrow. PME (5 mM) eliminates stromal cells and monocytes. Colony stimulatory factors were used at plateau concentrations. TGF-beta 1 (0.1 ng/ml) significantly (p < 0.05) augmented M-CSF induced macrophage colony forming units (CFU-M) by twofold to fourfold in 8/8 donors. In contrast, colonies stimulated by granulocyte-macrophage CSF (GM-CSF) (CFU-GM), were significantly decreased by TGF-beta 1. To determine if TGF-beta 1 was present in effective concentrations in vitro, cultures were performed with anti-TGF-beta 1. Anti-TGF-beta 1 decreased (p < 0.05) M-CSF induced colonies in 5/6 donors. The method of TGF-beta 1 enhancement was explored with antihuman CSF-1 receptor antibody. Antihuman CSF-1 receptor antibody resulted in comparable suppression of CFU-M resulting from both M-CSF and M-CSF + TGF-beta 1. These studies indicate that TGF-beta 1 directly enhances M-CSF activity by a mechanism other than upregulation of M-CSF receptors.