InVivoMAb anti-human/monkey CD20
Product Details
The 2H7 monoclonal antibody reacts with human and primate CD20. CD20 is a B cell-specific 33-37 kDa transmembrane protein which is also known as B-lymphocyte antigen, B1, and Bp35. CD20 plays roles in intracellular calcium regulation and B cell activation and is critical for an optimal B cell immune response against T-independent antigens. CD20 is first expressed after the induction of CD19 together with IgM during the pre-B to immature B cell transition in the bone marrow. It’s expression then increases during maturation with almost all mature B cells expressing some level of CD20. However, CD20 is not expressed by plasma blasts or plasma cells. CD20 is expressed by most B cell neoplasms, and is useful in diagnosing B cell lymphomas and leukemia. Many anti-CD20 monoclonal antibodies are currently being used to successfully treat leukemia, lymphomas, and various autoimmune diseases. The 2H7 monoclonal antibody has been shown to bind to an epitope in the large extracellular loop of human CD20.Specifications
| Isotype | Mouse IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2b isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human tonsillar B cells |
| Reported Applications |
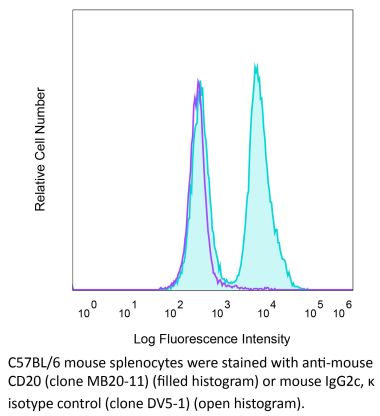
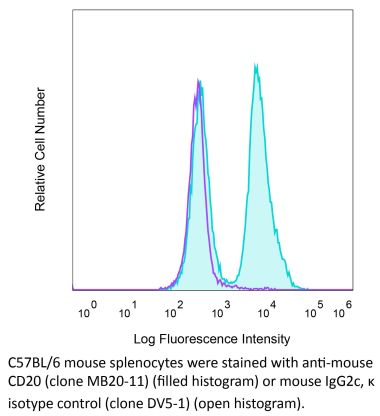
in vivo B cell depletion in hCD20 Tg mice Immunohistochemistry (frozen) Immunoprecipitation Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
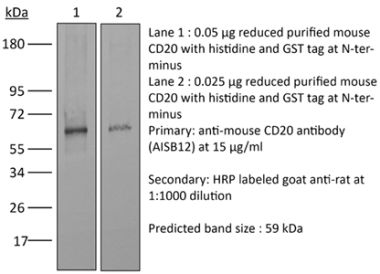
>95% Determined by SDS-PAGE |
| Sterility | 0.2 μM filtered |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_2687799 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
Flow Cytometry
Li, R., et al. (2015). "Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy" Sci Transl Med 7(310): 310ra166. PubMed
B cells are not limited to producing protective antibodies; they also perform additional functions relevant to both health and disease. However, the relative contribution of functionally distinct B cell subsets in human disease, the signals that regulate the balance between such subsets, and which of these subsets underlie the benefits of B cell depletion therapy (BCDT) are only partially elucidated. We describe a proinflammatory, granulocyte macrophage-colony stimulating factor (GM-CSF)-expressing human memory B cell subset that is increased in frequency and more readily induced in multiple sclerosis (MS) patients compared to healthy controls. In vitro, GM-CSF-expressing B cells efficiently activated myeloid cells in a GM-CSF-dependent manner, and in vivo, BCDT resulted in a GM-CSF-dependent decrease in proinflammatory myeloid responses of MS patients. A signal transducer and activator of transcription 5 (STAT5)- and STAT6-dependent mechanism was required for B cell GM-CSF production and reciprocally regulated the generation of regulatory IL-10-expressing B cells. STAT5/6 signaling was enhanced in B cells of untreated MS patients compared with healthy controls, and B cells reemerging in patients after BCDT normalized their STAT5/6 signaling as well as their GM-CSF/IL-10 cytokine secretion ratios. The diminished proinflammatory myeloid cell responses observed after BCDT persisted even as new B cells reconstituted. These data implicate a proinflammatory B cell/myeloid cell axis in disease and underscore the rationale for selective targeting of distinct B cell populations in MS and other human autoimmune diseases.
in vivo B cell depletion in hCD20 Tg mice
Hu, C., et al. (2013). "Combination treatment with anti-CD20 and oral anti-CD3 prevents and reverses autoimmune diabetes" Diabetes 62(8): 2849-2858. PubMed
Type 1 diabetes (T1D) is a T cell-mediated autoimmune disease, although B cells also play an important role in T1D development. Both T cell- and B cell-directed immunotherapies have shown efficacy in the prevention and reversal of T1D. However, whether the combined strategy of targeting both T and B cells could further improve therapeutic efficacy remains to be explored. We show that combined treatment with intravenous antihuman CD20 (hCD20) and oral anti-CD3 significantly delays diabetes development in prediabetic hCD20 transgenic NOD mice. More importantly, the combined treatment reverses diabetes in >60% of mice newly diagnosed with diabetes. Further mechanistic studies demonstrated that the addition of oral anti-CD3 to the B-cell depletion therapy synergistically enhances the suppressive function of regulatory T cells. Of note, the oral anti-CD3 treatment induced a fraction of interleukin (IL)-10-producing CD4 T cells in the small intestine through IL-10- and IL-27-producing dendritic cells. Thus, the findings demonstrate that combining anti-CD20 and oral anti-CD3 is superior to anti-CD20 monotherapy for restoring normoglycemia in diabetic NOD mice, providing important preclinical evidence for the optimization of B cell-directed therapy for T1D.
in vivo B cell depletion in hCD20 Tg mice
Xiang, Y., et al. (2012). "The dual effects of B cell depletion on antigen-specific T cells in BDC2.5NOD mice" J Immunol 188(10): 4747-4758. PubMed
B cells play a critical role in the pathogenesis of autoimmune diabetes. To investigate the mechanisms by which B cell depletion therapy attenuates islet beta cell loss and particularly to examine the effect of B cells on both diabetogenic and regulatory Ag-specific T cells, we generated a transgenic BDC2.5NOD mouse expressing human CD20 on B cells. This allowed us to deplete B cells for defined time periods and investigate the effect of B cell depletion on Ag-specific BDC2.5 T cells. We depleted B cells with anti-human CD20 Ab using a multiple injection protocol. We studied two time points, before and after B cell regeneration, to examine the effect on BDC2.5 T cell phenotype and functions that included antigenic response, cytokine profile, diabetogenicity, and suppressive function of regulatory T (T(reg)) cells. We found unexpectedly that B cell depletion induced transient aggressive behavior in BDC2.5 diabetogenic T cells and reduction in T(reg) cell number and function during the depletion period. However, after B cell reconstitution, we found that more regenerated B cells, particularly in the CD1d(-) fraction, expressed immune regulatory function. Our results suggest that the regenerated B cells are likely to be responsible for the therapeutic effect after B cell depletion. Our preclinical study also provides direct evidence that B cells regulate both pathogenic and T(reg) cell function, and this knowledge could explain the increased T cell responses to islet Ag after rituximab therapy in diabetic patients in a recent report and will be useful in design of future clinical protocols.
in vivo B cell depletion in hCD20 Tg mice
Keren, Z., et al. (2011). "B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging" Blood 117(11): 3104-3112. PubMed
Aging is associated with a decline in B-lymphopoiesis in the bone marrow and accumulation of long-lived B cells in the periphery. These changes decrease the body’s ability to mount protective antibody responses. We show here that age-related changes in the B lineage are mediated by the accumulating long-lived B cells. Thus, depletion of B cells in old mice was followed by expansion of multipotent primitive progenitors and common lymphoid progenitors, a revival of B-lymphopoiesis in the bone marrow, and generation of a rejuvenated peripheral compartment that enhanced the animal’s immune responsiveness to antigenic stimulation. Collectively, our results suggest that immunosenescence in the B-lineage is not irreversible and that depletion of the long-lived B cells in old mice rejuvenates the B-lineage and enhances immune competence.
in vivo B cell depletion in hCD20 Tg mice
Weber, M. S., et al. (2010). "B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity" Ann Neurol 68(3): 369-383. PubMed
OBJECTIVE: Clinical studies indicate that anti-CD20 B-cell depletion may be an effective multiple sclerosis (MS) therapy. We investigated mechanisms of anti-CD20-mediated immune modulation using 2 paradigms of experimental autoimmune encephalomyelitis (EAE). METHODS: Murine EAE was induced by recombinant myelin oligodendrocyte glycoprotein (rMOG), a model in which B cells are considered to contribute pathogenically, or MOG peptide (p)35-55, which does not require B cells. RESULTS: In EAE induced by rMOG, B cells became activated and, when serving as antigen-presenting cells (APCs), promoted differentiation of proinflammatory MOG-specific Th1 and Th17 cells. B-cell depletion prevented or reversed established rMOG-induced EAE, which was associated with less central nervous system (CNS) inflammation, elimination of meningeal B cells, and reduction of MOG-specific Th1 and Th17 cells. In contrast, in MOG p35-55-induced EAE, B cells did not become activated or efficiently polarize proinflammatory MOG-specific T cells, similar to naive B cells. In this setting, anti-CD20 treatment exacerbated EAE, and did not impede development of Th1 or Th17 cells. Irrespective of the EAE model used, B-cell depletion reduced the frequency of CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg), and increased the proinflammatory polarizing capacity of remaining myeloid APCs. INTERPRETATION: Our study highlights distinct roles for B cells in CNS autoimmunity. Clinical benefit from anti-CD20 treatment may relate to inhibition of proinflammatory B cell APC function. In certain clinical settings, however, elimination of unactivated B cells, which participate in regulation of T cells and other APC, may be undesirable. Differences in immune responses to MOG protein and peptide may be important considerations when choosing an EAE model for testing novel B cell-targeting agents for MS.
Immunohistochemistry (frozen)
Mack, C. L., et al. (2004). "Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation" Pediatr Res 56(1): 79-87. PubMed
A proposed mechanism in the pathogenesis of biliary atresia involves an initial virus-induced, progressive T cell-mediated inflammatory obliteration of bile ducts. The aim of this study was to characterize the inflammatory environment present within the liver of infants with biliary atresia to gain insight into the role of a primary immune-mediated process versus a nonspecific secondary response to biliary obstruction. Frozen liver tissue obtained from patients with biliary atresia, neonatal giant cell hepatitis, total parenteral nutrition (TPN)-related cholestasis, choledochal cysts, and normal control subjects was used for fluorescent immunohistochemistry studies of cellular infiltrates, cytokine mRNA expression, and in situ hybridization for localization of cytokine-producing cells. Immunohistochemistry revealed increases in CD8(+) and CD4(+) T cells and Kupffer cells (CD68(+)) in the portal tracts of biliary atresia. Reverse transcription-PCR analysis of biliary atresia tissue showed a Th1-type cytokine profile with expression of IL-2, interferon-gamma, tumor necrosis factor-alpha, and IL-12. This profile was not seen in normal, neonatal hepatitis or choledochal cyst livers but was present in TPN-related cholestasis. In situ hybridization revealed that the Th1 cytokine-producing cells were located in the portal tracts in biliary atresia and in the parenchyma of TPN-related cholestasis. A distinctive portal tract inflammatory environment is present in biliary atresia, involving CD4(+) Th1 cell-mediated immunity. The absence of similar inflammation in other pediatric cholestatic conditions suggests that the portal tract inflammation in biliary atresia is not a secondary response to cholestasis but rather indicates a specific immune response involved in the pathogenesis of biliary atresia.
Immunoprecipitation
Polyak, M. J. and J. P. Deans. (2002). "Alanine-170 and proline-172 are critical determinants for extracellular CD20 epitopes; heterogeneity in the fine specificity of CD20 monoclonal antibodies is defined by additional requirements imposed by both amino acid sequence and quaternary structure" Blood 99(9): 3256-3262. PubMed
In vivo ablation of malignant B cells can be achieved using antibodies directed against the CD20 antigen. Fine specificity differences among CD20 monoclonal antibodies (mAbs) are assumed not to be a factor in determining their efficacy because evidence from antibody-blocking studies indicates limited epitope diversity with only 2 overlapping extracellular CD20 epitopes. However, in this report a high degree of heterogeneity among antihuman CD20 mAbs is demonstrated. Mutation of alanine and proline at positions 170 and 172 (AxP) (single-letter amino acid codes; x indicates the identical amino acid at the same position in the murine and human CD20 sequences) in human CD20 abrogated the binding of all CD20 mAbs tested. Introduction of AxP into the equivalent positions in the murine sequence, which is not otherwise recognized by antihuman CD20 mAbs, fully reconstituted the epitope recognized by B1, the prototypic anti-CD20 mAb. 2H7, a mAb previously thought to recognize the same epitope as B1, did not recognize the murine AxP mutant. Reconstitution of the 2H7 epitope was achieved with additional mutations replacing VDxxD in the murine sequence for INxxN (positions 162-166 in the human sequence). The integrity of the 2H7 epitope, unlike that of B1, further depends on the maintenance of CD20 in an oligomeric complex. The majority of 16 antihuman CD20 mAbs tested, including rituximab, bound to murine CD20 containing the AxP mutations. Heterogeneity in the fine specificity of these antibodies was indicated by marked differences in their ability to induce homotypic cellular aggregation and translocation of CD20 to a detergent-insoluble membrane compartment previously identified as lipid rafts.