InVivoMAb anti-human CD8α
Product Details
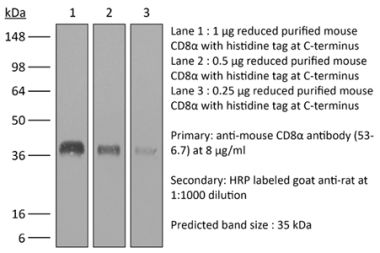
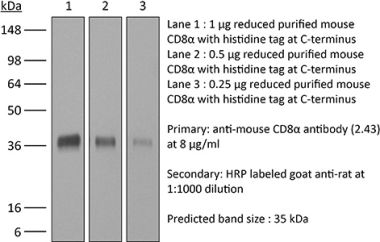
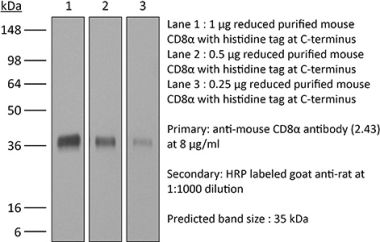
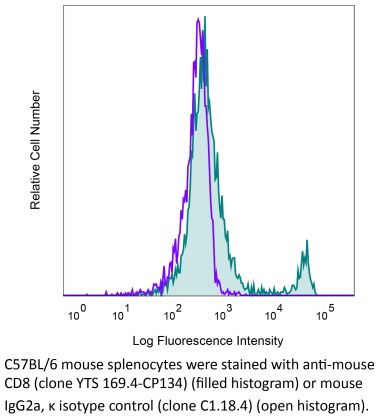
The OKT-8 monoclonal antibody reacts with human CD8α. The CD8 antigen is a transmembrane glycoprotein that acts as a co-receptor for the T cell receptor (TCR). Like the TCR, CD8 binds to class I MHC molecules displayed by antigen presenting cells (APC). CD8 is primarily expressed on the surface of cytotoxic T cells, but can also be found on thymocytes, natural killer cells, and some dendritic cell subsets. CD8 most commonly exists as a heterodimer composed of one CD8α and one CD8β chain however, it can also exist as a homodimer composed of two CD8α chains. Both the CD8α and CD8β chains share significant homology to immunoglobulin variable light chains. The molecular weight of each CD8 chain is approximately 34 kDa. The OKT-8 antibody does not block the binding of the anti-human CD8α antibodies RPA-T8 and HIT8a.Specifications
| Isotype | Mouse IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG2a isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 6.0T Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human CD8α |
| Reported Applications | in vivo CD8+ T cell depletion in humanized mice |
| Formulation |
PBS, pH 6.0 0.01% Tween Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107673 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo CD8+ T cell depletion in humanized mice
Chijioke, O., et al. (2015). "Role of the 2B4 Receptor in CD8+ T-Cell-Dependent Immune Control of Epstein-Barr Virus Infection in Mice With Reconstituted Human Immune System Components" J Infect Dis 212(5): 803-807. PubMed
Patients with X-linked lymphoproliferative (XLP) disease due to deficiency in the adaptor molecule signaling lymphocytic activation molecule-associated protein (SAP) are highly susceptible to one specific viral pathogen, the Epstein-Barr virus (EBV). This susceptibility might result from impaired CD8(+) T-cell and natural killer cell responses to EBV infection in these patients. We demonstrate that antibody blocking of the SAP-dependent 2B4 receptor is sufficient to induce XLP-like aggravation of EBV disease in mice with reconstituted human immune system components. CD8(+) T cells require 2B4 for EBV-specific immune control, because 2B4 blockade after CD8(+) T-cell depletion did not further aggravate symptoms of EBV infection.
in vivo CD8+ T cell depletion in humanized mice
Billerbeck, E., et al. (2013). "Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice" J Immunol 191(4): 1753-1764. PubMed
Humanized mice have emerged as a promising model to study human immunity in vivo. Although they are susceptible to many pathogens exhibiting an almost exclusive human tropism, human immune responses to infection remain functionally impaired. It has recently been demonstrated that the expression of HLA molecules improves human immunity to lymphotropic virus infections in humanized mice. However, little is known about the extent of functional human immune responses in nonlymphoid tissues, such as in the liver, and the role of HLA expression in this context. Therefore, we analyzed human antiviral immunity in humanized mice during a hepatotropic adenovirus infection. We compared immune responses of conventional humanized NOD SCID IL-2Rgamma-deficient (NSG) mice to those of a novel NOD SCID IL-2Rgamma-deficient strain transgenic for both HLA-A*0201 and a chimeric HLA-DR*0101 molecule. Using a firefly luciferase-expressing adenovirus and in vivo bioluminescence imaging, we demonstrate a human T cell-dependent partial clearance of adenovirus-infected cells from the liver of HLA-transgenic humanized mice. This correlated with liver infiltration and activation of T cells, as well as the detection of Ag-specific humoral and cellular immune responses. When infected with a hepatitis C virus NS3-expressing adenovirus, HLA-transgenic humanized mice mounted an HLA-A*0201-restricted hepatitis C virus NS3-specific CD8(+) T cell response. In conclusion, our study provides evidence for the generation of partial functional antiviral immune responses against a hepatotropic pathogen in humanized HLA-transgenic mice. The adenovirus reporter system used in our study may serve as simple in vivo method to evaluate future strategies for improving human intrahepatic immune responses in humanized mice.
in vivo CD8+ T cell depletion in humanized mice
Chijioke, O., et al. (2013). "Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection" Cell Rep 5(6): 1489-1498. PubMed
Primary infection with the human oncogenic Epstein-Barr virus (EBV) can result in infectious mononucleosis (IM), a self-limiting disease caused by massive lymphocyte expansion that predisposes for the development of distinct EBV-associated lymphomas. Why some individuals experience this symptomatic primary EBV infection, whereas the majority acquires the virus asymptomatically, remains unclear. Using a mouse model with reconstituted human immune system components, we show that depletion of human natural killer (NK) cells enhances IM symptoms and promotes EBV-associated tumorigenesis mainly because of a loss of immune control over lytic EBV infection. These data suggest that failure of innate immune control by human NK cells augments symptomatic lytic EBV infection, which drives lymphocyte expansion and predisposes for EBV-associated malignancies.