InVivoMAb anti-mouse IL-17A
Product Details
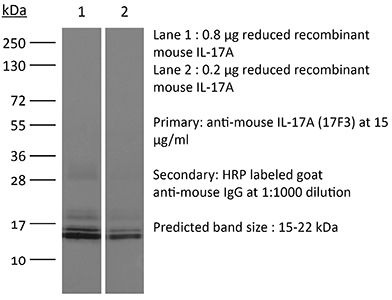
The 17F3 monoclonal antibody reacts with mouse IL-17A a 15-20 kDa cytokine expressed by Th17 cells, γδ T cells, iNKT cells, NK cells, LTi cells, neutrophils, and intestinal Paneth cells. IL-17A has pleiotropic effects in immunoregulation and inflammation. It plays an important role in anti-microbial and chronic inflammation by inducing cytokine and chemokine production, neutrophil influx, and the production of antibacterial peptides but it is also an inflammatory mediator in the development of autoimmune diseases including rheumatoid arthritis, asthma, multiple sclerosis, and psoriasis. The 17F3 antibody has been shown to neutralize IL-17A in vivo.Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse IL-17A cross-linked to OVA |
| Reported Applications | in vivo IL-17A neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950102 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo IL-17A neutralization
Faraco, G., et al. (2018). "Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response" Nat Neurosci 21(2): 240-249. PubMed
A diet rich in salt is linked to an increased risk of cerebrovascular diseases and dementia, but it remains unclear how dietary salt harms the brain. We report that, in mice, excess dietary salt suppresses resting cerebral blood flow and endothelial function, leading to cognitive impairment. The effect depends on expansion of TH17 cells in the small intestine, resulting in a marked increase in plasma interleukin-17 (IL-17). Circulating IL-17, in turn, promotes endothelial dysfunction and cognitive impairment by the Rho kinase-dependent inhibitory phosphorylation of endothelial nitric oxide synthase and reduced nitric oxide production in cerebral endothelial cells. The findings reveal a new gut-brain axis linking dietary habits to cognitive impairment through a gut-initiated adaptive immune response compromising brain function via circulating IL-17. Thus, the TH17 cell-IL-17 pathway is a putative target to counter the deleterious brain effects induced by dietary salt and other diseases associated with TH17 polarization.
in vivo IL-17A neutralization
Xiong, H., et al. (2016). "Innate Lymphocyte/Ly6C Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance" Cell. doi : 10.1016/j.cell.2016.03.017. PubMed
Increasing antibiotic resistance among bacterial pathogens has rendered some infections untreatable with available antibiotics. Klebsiella pneumoniae, a bacterial pathogen that has acquired high-level antibiotic resistance, is a common cause of pulmonary infections. Optimal clearance of K. pneumoniae from the host lung requires TNF and IL-17A. Herein, we demonstrate that inflammatory monocytes are rapidly recruited to the lungs of K. pneumoniae-infected mice and produce TNF, which markedly increases the frequency of IL-17-producing innate lymphoid cells. While pulmonary clearance of K. pneumoniae is preserved in neutrophil-depleted mice, monocyte depletion or TNF deficiency impairs IL-17A-dependent resolution of pneumonia. Monocyte-mediated bacterial uptake and killing is enhanced by ILC production of IL-17A, indicating that innate lymphocytes engage in a positive-feedback loop with monocytes that promotes clearance of pneumonia. Innate immune defense against a highly antibiotic-resistant bacterial pathogen depends on crosstalk between inflammatory monocytes and innate lymphocytes that is mediated by TNF and IL-17A.
in vivo IL-17A neutralization
Naik, S., et al. (2015). "Commensal-dendritic-cell interaction specifies a unique protective skin immune signature" Nature 520(7545): 104-108. PubMed
The skin represents the primary interface between the host and the environment. This organ is also home to trillions of microorganisms that play an important role in tissue homeostasis and local immunity. Skin microbial communities are highly diverse and can be remodelled over time or in response to environmental challenges. How, in the context of this complexity, individual commensal microorganisms may differentially modulate skin immunity and the consequences of these responses for tissue physiology remains unclear. Here we show that defined commensals dominantly affect skin immunity and identify the cellular mediators involved in this specification. In particular, colonization with Staphylococcus epidermidis induces IL-17A(+) CD8(+) T cells that home to the epidermis, enhance innate barrier immunity and limit pathogen invasion. Commensal-specific T-cell responses result from the coordinated action of skin-resident dendritic cell subsets and are not associated with inflammation, revealing that tissue-resident cells are poised to sense and respond to alterations in microbial communities. This interaction may represent an evolutionary means by which the skin immune system uses fluctuating commensal signals to calibrate barrier immunity and provide heterologous protection against invasive pathogens. These findings reveal that the skin immune landscape is a highly dynamic environment that can be rapidly and specifically remodelled by encounters with defined commensals, findings that have profound implications for our understanding of tissue-specific immunity and pathologies.
in vivo IL-17A neutralization
Sell, S., et al. (2015). "Control of murine cytomegalovirus infection by gammadelta T cells" PLoS Pathog 11(2): e1004481. PubMed
Infections with cytomegalovirus (CMV) can cause severe disease in immunosuppressed patients and infected newborns. Innate as well as cellular and humoral adaptive immune effector functions contribute to the control of CMV in immunocompetent individuals. None of the innate or adaptive immune functions are essential for virus control, however. Expansion of gammadelta T cells has been observed during human CMV (HCMV) infection in the fetus and in transplant patients with HCMV reactivation but the protective function of gammadelta T cells under these conditions remains unclear. Here we show for murine CMV (MCMV) infections that mice that lack CD8 and CD4 alphabeta-T cells as well as B lymphocytes can control a MCMV infection that is lethal in RAG-1(-/-) mice lacking any T- and B-cells. gammadelta T cells, isolated from infected mice can kill MCMV infected target cells in vitro and, importantly, provide long-term protection in infected RAG-1(-/-) mice after adoptive transfer. gammadelta T cells in MCMV infected hosts undergo a prominent and long-lasting phenotypic change most compatible with the view that the majority of the gammadelta T cell population persists in an effector/memory state even after resolution of the acute phase of the infection. A clonotypically focused Vgamma1 and Vgamma2 repertoire was observed at later stages of the infection in the organs where MCMV persists. These findings add gammadelta T cells as yet another protective component to the anti-CMV immune response. Our data provide clear evidence that gammadelta T cells can provide an effective control mechanism of acute CMV infections, particularly when conventional adaptive immune mechanisms are insufficient or absent, like in transplant patient or in the developing immune system in utero. The findings have implications in the stem cell transplant setting, as antigen recognition by gammadelta T cells is not MHC-restricted and dual reactivity against CMV and tumors has been described.
in vivo IL-17A neutralization
Coffelt, S. B., et al. (2015). "IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis" Nature 522(7556): 345-348. PubMed
Metastatic disease remains the primary cause of death for patients with breast cancer. The different steps of the metastatic cascade rely on reciprocal interactions between cancer cells and their microenvironment. Within this local microenvironment and in distant organs, immune cells and their mediators are known to facilitate metastasis formation. However, the precise contribution of tumour-induced systemic inflammation to metastasis and the mechanisms regulating systemic inflammation are poorly understood. Here we show that tumours maximize their chance of metastasizing by evoking a systemic inflammatory cascade in mouse models of spontaneous breast cancer metastasis. We mechanistically demonstrate that interleukin (IL)-1beta elicits IL-17 expression from gamma delta (gammadelta) T cells, resulting in systemic, granulocyte colony-stimulating factor (G-CSF)-dependent expansion and polarization of neutrophils in mice bearing mammary tumours. Tumour-induced neutrophils acquire the ability to suppress cytotoxic T lymphocytes carrying the CD8 antigen, which limit the establishment of metastases. Neutralization of IL-17 or G-CSF and absence of gammadelta T cells prevents neutrophil accumulation and downregulates the T-cell-suppressive phenotype of neutrophils. Moreover, the absence of gammadelta T cells or neutrophils profoundly reduces pulmonary and lymph node metastases without influencing primary tumour progression. Our data indicate that targeting this novel cancer-cell-initiated domino effect within the immune system–the gammadelta T cell/IL-17/neutrophil axis–represents a new strategy to inhibit metastatic disease.
in vivo IL-17A neutralization
Xin, L., et al. (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677. PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo IL-17A neutralization
Kulcsar, K. A., et al. (2014). "Interleukin 10 modulation of pathogenic Th17 cells during fatal alphavirus encephalomyelitis" Proc Natl Acad Sci U S A 111(45): 16053-16058. PubMed
Mosquito-borne alphaviruses are important causes of epidemic encephalomyelitis. Neuronal cell death during fatal alphavirus encephalomyelitis is immune-mediated; however, the types of cells involved and their regulation have not been determined. We show that the virus-induced inflammatory response was accompanied by production of the regulatory cytokine IL-10, and in the absence of IL-10, paralytic disease occurred earlier and mice died faster. To determine the reason for accelerated disease in the absence of IL-10, immune responses in the CNS of IL-10(-/-) and wild-type (WT) mice were compared. There were no differences in the amounts of brain inflammation or peak virus replication; however, IL-10(-/-) animals had accelerated and increased infiltration of CD4(+)IL-17A(+) and CD4(+)IL-17A(+)IFNgamma(+) cells compared with WT animals. Th17 cells infiltrating the brain demonstrated a pathogenic phenotype with the expression of the transcription factor, Tbet, and the production of granzyme B, IL-22, and GM-CSF, with greater production of GM-CSF in IL-10(-/-) mice. Therefore, in fatal alphavirus encephalomyelitis, pathogenic Th17 cells enter the CNS at the onset of neurologic disease and, in the absence of IL-10, appear earlier, develop into Th1/Th17 cells more often, and have greater production of GM-CSF. This study demonstrates a role for pathogenic Th17 cells in fatal viral encephalitis.
in vivo IL-17A neutralization
Khmaladze, I., et al. (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678. PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vivo IL-17A neutralization
Ermann, J., et al. (2014). "Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet-/-.Rag2-/- (TRUC) mice" Proc Natl Acad Sci U S A 111(25): E2559-2566. PubMed
T-bet(-/-).Rag2(-/-) (TRUC) mice spontaneously develop microbiota-driven, TNF-mediated large bowel inflammation that resembles human ulcerative colitis. We show here that IL-23 and IL-1-dependent secretion of IL-17A by innate lymphoid cells (ILCs; defined as CD45(+)lin(-)Thy1(hi)NKp46(-)) is a second critical pathway in this model. Using an in vitro coculture system of bone marrow-derived dendritic cells (DCs) and freshly isolated FACS-purified ILCs, we demonstrate that IL-23 and IL-1 secreted by DCs in response to microbial stimulation work together to induce IL-17A production by ILCs. TNF is not required for IL-17A secretion by ILCs in vitro but synergizes with IL-17A to induce the expression of neutrophil-attracting chemokines. Upstream, activation of the IL-23/IL-17A axis is regulated by nucleotide-binding oligomerization domain containing (Nod)/receptor-interacting serine-threonine kinase 2 (Ripk2) signals in DCs. Genetic ablation of the Nod/Ripk2 signaling pathway protects TRUC mice from developing colitis without affecting the colitogenicity of the intestinal microbiota. Our data provide insight into the complex network of interactions between IL-17A-secreting ILCs and other components of the innate immune system in the development of colitis.
in vivo IL-17A neutralization
Uddin, M. N., et al. (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953. PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo IL-17A neutralization
Gladiator, A., et al. (2013). "Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection" J Immunol 190(2): 521-525. PubMed
IL-17-mediated immunity has emerged as a crucial host defense mechanism against fungal infections. Although Th cells are generally thought to act as the major source of IL-17 in response to Candida albicans, we show that fungal control is mediated by IL-17-secreting innate lymphoid cells (ILCs) and not by Th17 cells. By using a mouse model of oropharyngeal candidiasis we found that IL-17A and IL-17F, which are both crucial for pathogen clearance, are produced promptly upon infection in an IL-23-dependent manner, and that ILCs in the oral mucosa are the main source for these cytokines. Ab-mediated depletion of ILCs in RAG1-deficient mice or ILC deficiency in retinoic acid-related orphan receptor c(-/-) mice resulted in a complete failure to control the infection. Taken together, our data uncover the cellular basis for the IL-23/IL-17 axis, which acts right at the onset of infection when it is most needed for fungal control and host protection.
in vivo IL-17A neutralization
Gonzalez-Lombana, C., et al. (2013). "IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection" PLoS Pathog 9(3): e1003243. PubMed
Leishmaniasis, resulting from infection with the protozoan parasite Leishmania, consists of a wide spectrum of clinical manifestations, from healing cutaneous lesions to fatal visceral infections. A particularly severe form of cutaneous leishmaniasis, termed mucosal leishmaniasis, exhibits decreased IL-10 levels and an exaggerated inflammatory response that perpetuates the disease. Using a mouse model of leishmaniasis, we investigated what cytokines contribute to increased pathology when IL-10-mediated regulation is absent. Leishmania major infected C57BL/6 mice lacking IL-10 regulation developed larger lesions than controls, but fewer parasites. Both IFN-gamma and IL-17 levels were substantially elevated in mice lacking the capacity to respond to IL-10. IFN-gamma promoted an increased infiltration of monocytes, while IL-17 contributed to an increase in neutrophils. Surprisingly, however, we found that IFN-gamma did not contribute to increased pathology, but instead regulated the IL-17 response. Thus, blocking IFN-gamma led to a significant increase in IL-17, neutrophils and disease. Similarly, the production of IL-17 by cells from leishmaniasis patients was also regulated by IL-10 and IFN-gamma. Additional studies found that the IL-1 receptor was required for both the IL-17 response and increased pathology. Therefore, we propose that regulating IL-17, possibly by downregulating IL-1beta, may be a useful approach for controlling immunopathology in leishmaniasis.
in vivo IL-17A neutralization
Berger, H., et al. (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590. PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vivo IL-17A neutralization
Valdez, P. A., et al. (2012). "Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells" Immunity 36(4): 668-679. PubMed
T helper 17 (Th17) cells play an important role in mucosal host defense through production of the signature cytokines IL-17 and IL-22. Prostaglandin E2 (PGE2) has been shown to enhance IL-17 production by mature Th17 cells. However, when present during Th17 cell differentiation, we found that PGE2 inhibited the transcription factor IRF4 and suppressed production of IL-17 but not IL-22. We show that IRF4 was required for IL-17 expression but inhibited IL-22 expression, highlighting the potential for discordant regulation of these two cytokines in Th17 cells. The pathogenic fungus Cryptococcus neoformans produces PGE2, and we found that it uses PGE2- and IRF4-dependent mechanisms to specifically inhibit induction of IL-17 during Th17 cell differentiation. Blockade of host PGE2 during infection led to increased IL-17 production from CD4(+) T cells and increased survival of mice. These findings suggest that host- or pathogen-derived PGE2 can act directly on Th17 cells during differentiation to inhibit IL-17-dependent antimicrobial responses.