InVivoMAb anti-mouse/human/rat/monkey/hamster/canine/bovine TGF-β
Product Details
The 1D11.16.8 monoclonal antibody reacts with mouse, human, rat, monkey, hamster, canine and bovine TGF-β (transforming growth factor beta) isoforms 1, 2 and 3. TGF-β is a multifunctional cytokine that regulates the proliferation of epithelial cells, endothelial cells, fibroblasts, neurons, lymphoid cells including T lymphocytes and NK cells, and other hematopoietic cell types. TGF-β also regulates the activities of activated macrophages and the development of regulatory T cells. Additionally, TGF-β plays roles in immune function, tissue remodeling and wound repair. TGF-β exists as five highly similar isoforms (TGF-β 1-5) with homologies of 70-80%. TGF-β1 is synthesized by the enzymatic cleavage of a long precursor TGF-β1 polypeptide encoded by the TGFB1 gene which yields the mature protein and the Latency Associated Peptide (LAP). The LAP and mature TGF-β1 non-covalently associate during secretion. TGF-β is ubiquitously expressed by many cell types including macrophages and platelets which express high levels of TGF-β. TGF-β signaling has been shown to plays roles in cancer, autoimmune diseases, asthma, heart disease, and diabetes. Its importance is illustrated by TGF-β knockout mice which show defects in hematopoiesis and endothelial differentiation, and die of overwhelming inflammation. The 1D11.16.8 monoclonal antibody is a neutralizing antibody.Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
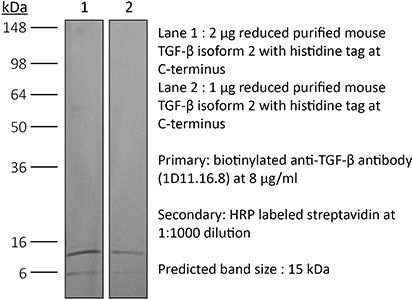
| Immunogen | Bovine TGFβ isoform 2 |
| Reported Applications |
in vivo TGFβ neutralization in vitro TGFβ neutralization Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107757 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo TGFβ neutralization
Komai, T., et al. (2018). "Transforming Growth Factor-beta and Interleukin-10 Synergistically Regulate Humoral Immunity via Modulating Metabolic Signals" Front Immunol 9: 1364. PubMed
Inhibitory cytokines, such as transforming growth factor-beta (TGF-beta) and interleukin-10 (IL-10), are humoral factors involved in the suppressive function of regulatory T cells and play critical roles in maintaining immune homeostasis. However, TGF-beta and IL-10 also have pleiotropic effects and induce humoral immune responses depending on conditions, and thus their therapeutic application to autoimmune diseases remains limited. Here, we show that a combination of TGF-beta and IL-10, but not single cytokine, is required to suppress B cell activation induced by toll-like receptor (TLR) stimulation. In in vivo analyses, the simultaneous presence of TGF-beta and IL-10 effectively suppressed TLR-mediated antigen-specific immune responses and ameliorated pathologies in imiquimod (TLR7 agonist)-induced lupus model and lupus-prone MRL/lpr mice. Intriguingly, TGF-beta and IL-10 synergistically modulated transcriptional programs and suppressed cellular energetics of both glycolysis and oxidative phosphorylation via inhibition of the mammalian target of rapamycin complex 1 (mTORC1)/S6 kinase 1 (S6K1) pathway in TLR-stimulated B cells. On the other hand, enhancement of mTOR signaling and mitochondrial biosynthesis in TLR-stimulated B cells counteracted the synergistic inhibitory effects. The inhibitory cytokine synergy of TGF-beta and IL-10 via suppression of energy metabolism was also observed in human TLR-stimulated B cells. There is increasing evidence supporting the importance of adequate metabolic signals in various immune cells to exert their immune function. In this study, we have shown that a previously unrecognized synergy of inhibitory cytokines regulates systemic humoral immune responses via modulating immunometabolism in B cells. Our findings indicate that inhibition of B cell metabolism mediated by two synergistic cytokines contributes to the induction of immune tolerance and could be a new therapeutic strategy for autoimmune diseases such as systemic lupus erythematosus.
in vivo TGFβ neutralization
Clemente-Casares, X., et al. (2016). "Expanding antigen-specific regulatory networks to treat autoimmunity" Nature 530(7591): 434-440. PubMed
Regulatory T cells hold promise as targets for therapeutic intervention in autoimmunity, but approaches capable of expanding antigen-specific regulatory T cells in vivo are currently not available. Here we show that systemic delivery of nanoparticles coated with autoimmune-disease-relevant peptides bound to major histocompatibility complex class II (pMHCII) molecules triggers the generation and expansion of antigen-specific regulatory CD4(+) T cell type 1 (TR1)-like cells in different mouse models, including mice humanized with lymphocytes from patients, leading to resolution of established autoimmune phenomena. Ten pMHCII-based nanomedicines show similar biological effects, regardless of genetic background, prevalence of the cognate T-cell population or MHC restriction. These nanomedicines promote the differentiation of disease-primed autoreactive T cells into TR1-like cells, which in turn suppress autoantigen-loaded antigen-presenting cells and drive the differentiation of cognate B cells into disease-suppressing regulatory B cells, without compromising systemic immunity. pMHCII-based nanomedicines thus represent a new class of drugs, potentially useful for treating a broad spectrum of autoimmune conditions in a disease-specific manner.
in vivo TGFβ neutralization
Manlove, L. S., et al. (2015). "Adaptive Immunity to Leukemia Is Inhibited by Cross-Reactive Induced Regulatory T Cells" J Immunol . PubMed
BCR-ABL+ acute lymphoblastic leukemia patients have transient responses to current therapies. However, the fusion of BCR to ABL generates a potential leukemia-specific Ag that could be a target for immunotherapy. We demonstrate that the immune system can limit BCR-ABL+ leukemia progression although ultimately this immune response fails. To address how BCR-ABL+ leukemia escapes immune surveillance, we developed a peptide: MHC class II tetramer that labels endogenous BCR-ABL-specific CD4+ T cells. Naive mice harbored a small population of BCR-ABL-specific T cells that proliferated modestly upon immunization. The small number of naive BCR-ABL-specific T cells was due to negative selection in the thymus, which depleted BCR-ABL-specific T cells. Consistent with this observation, we saw that BCR-ABL-specific T cells were cross-reactive with an endogenous peptide derived from ABL. Despite this cross-reactivity, the remaining population of BCR-ABL reactive T cells proliferated upon immunization with the BCR-ABL fusion peptide and adjuvant. In response to BCR-ABL+ leukemia, BCR-ABL-specific T cells proliferated and converted into regulatory T (Treg) cells, a process that was dependent on cross-reactivity with self-antigen, TGF-beta1, and MHC class II Ag presentation by leukemic cells. Treg cells were critical for leukemia progression in C57BL/6 mice, as transient Treg cell ablation led to extended survival of leukemic mice. Thus, BCR-ABL+ leukemia actively suppresses antileukemia immune responses by converting cross-reactive leukemia-specific T cells into Treg cells.
in vitro TGFβ neutralization
Bodogai, M., et al. (2015). "Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells" Cancer Res 75(17): 3456-3465. PubMed
Myeloid-derived suppressive cells (MDSC) have been reported to promote metastasis, but the loss of cancer-induced B cells/B regulatory cells (tBreg) can block metastasis despite MDSC expansion in cancer. Here, using multiple murine tumor models and human MDSC, we show that MDSC populations that expand in cancer have only partially primed regulatory function and limited prometastatic activity unless they are fully educated by tBregs. Cancer-induced tBregs directly activate the regulatory function of both the monocyte and granulocyte subpopulations of MDSC, relying, in part, on TgfbetaR1/TgfbetaR2 signaling. MDSC fully educated in this manner exhibit an increased production of reactive oxygen species and NO and more efficiently suppress CD4(+) and CD8(+) T cells, thereby promoting tumor growth and metastasis. Thus, loss of tBregs or TgfbetaR deficiency in MDSC is sufficient to disable their suppressive function and to block metastasis. Overall, our data indicate that cancer-induced B cells/B regulatory cells are important regulators of the immunosuppressive and prometastatic functions of MDSC.
in vitro TGFβ neutralization
Choi, Y. S., et al. (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990. PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vivo TGFβ neutralization
Greco, S. H., et al. (2015). "TGF-beta Blockade Reduces Mortality and Metabolic Changes in a Validated Murine Model of Pancreatic Cancer Cachexia" PLoS One 10(7): e0132786. PubMed
Cancer cachexia is a debilitating condition characterized by a combination of anorexia, muscle wasting, weight loss, and malnutrition. This condition affects an overwhelming majority of patients with pancreatic cancer and is a primary cause of cancer-related death. However, few, if any, effective therapies exist for both treatment and prevention of this syndrome. In order to develop novel therapeutic strategies for pancreatic cancer cachexia, appropriate animal models are necessary. In this study, we developed and validated a syngeneic, metastatic, murine model of pancreatic cancer cachexia. Using our model, we investigated the ability of transforming growth factor beta (TGF-beta) blockade to mitigate the metabolic changes associated with cachexia. We found that TGF-beta inhibition using the anti-TGF-beta antibody 1D11.16.8 significantly improved overall mortality, weight loss, fat mass, lean body mass, bone mineral density, and skeletal muscle proteolysis in mice harboring advanced pancreatic cancer. Other immunotherapeutic strategies we employed were not effective. Collectively, we validated a simplified but useful model of pancreatic cancer cachexia to investigate immunologic treatment strategies. In addition, we showed that TGF-beta inhibition can decrease the metabolic changes associated with cancer cachexia and improve overall survival.
in vivo TGFβ neutralization
Leon, B., et al. (2014). "FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability" Nat Commun 5: 3495. PubMed
Here, we test the role of FoxP3(+) regulatory T cells (Tregs) in controlling T follicular helper (Tfh) and germinal centre (GC) B-cell responses to influenza. In contrast to the idea that Tregs suppress T-cell responses, we find that Treg depletion severely reduces the Tfh cell response to influenza virus. Furthermore, Treg depletion prevents the accumulation of influenza-specific GCs. These effects are not due to alterations in TGFbeta availability or a precursor-progeny relationship between Tregs and Tfh cells, but are instead mediated by increased availability of IL-2, which suppresses the differentiation of Tfh cells and as a consequence, compromises the GC B response. Thus, Tregs promote influenza-specific GC responses by preventing excessive IL-2 signalling, which suppresses Tfh cell differentiation.
in vivo TGFβ neutralization
Li, W., et al. (2014). "Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis" J Clin Invest 124(2): 755-767. PubMed
TGF-beta is essential for vascular development; however, excess TGF-beta signaling promotes thoracic aortic aneurysm and dissection in multiple disorders, including Marfan syndrome. Since the pathology of TGF-beta overactivity manifests primarily within the arterial media, it is widely assumed that suppression of TGF-beta signaling in vascular smooth muscle cells will ameliorate aortic disease. We tested this hypothesis by conditional inactivation of Tgfbr2, which encodes the TGF-beta type II receptor, in smooth muscle cells of postweanling mice. Surprisingly, the thoracic aorta rapidly thickened, dilated, and dissected in these animals. Tgfbr2 disruption predictably decreased canonical Smad signaling, but unexpectedly increased MAPK signaling. Type II receptor-independent effects of TGF-beta and pathological responses by nonrecombined smooth muscle cells were excluded by serologic neutralization. Aortic disease was caused by a perturbed contractile apparatus in medial cells and growth factor production by adventitial cells, both of which resulted in maladaptive paracrine interactions between the vessel wall compartments. Treatment with rapamycin restored a quiescent smooth muscle phenotype and prevented dissection. Tgfbr2 disruption in smooth muscle cells also accelerated aneurysm growth in a murine model of Marfan syndrome. Our data indicate that basal TGF-beta signaling in smooth muscle promotes postnatal aortic wall homeostasis and impedes disease progression.
in vivo TGFβ neutralization, in vitro TGFβ neutralization
Worthington, J. J., et al. (2013). "Loss of the TGFbeta-activating integrin alphavbeta8 on dendritic cells protects mice from chronic intestinal parasitic infection via control of type 2 immunity" PLoS Pathog 9(10): e1003675. PubMed
Chronic intestinal parasite infection is a major global health problem, but mechanisms that promote chronicity are poorly understood. Here we describe a novel cellular and molecular pathway involved in the development of chronic intestinal parasite infection. We show that, early during development of chronic infection with the murine intestinal parasite Trichuris muris, TGFbeta signalling in CD4+ T-cells is induced and that antibody-mediated inhibition of TGFbeta function results in protection from infection. Mechanistically, we find that enhanced TGFbeta signalling in CD4+ T-cells during infection involves expression of the TGFbeta-activating integrin alphavbeta8 by dendritic cells (DCs), which we have previously shown is highly expressed by a subset of DCs in the intestine. Importantly, mice lacking integrin alphavbeta8 on DCs were completely resistant to chronic infection with T. muris, indicating an important functional role for integrin alphavbeta8-mediated TGFbeta activation in promoting chronic infection. Protection from infection was dependent on CD4+ T-cells, but appeared independent of Foxp3+ Tregs. Instead, mice lacking integrin alphavbeta8 expression on DCs displayed an early increase in production of the protective type 2 cytokine IL-13 by CD4+ T-cells, and inhibition of this increase by crossing mice to IL-4 knockout mice restored parasite infection. Our results therefore provide novel insights into how type 2 immunity is controlled in the intestine, and may help contribute to development of new therapies aimed at promoting expulsion of gut helminths.
in vivo TGFβ neutralization
Ring, S., et al. (2013). "Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice" J Immunol 191(6): 2938-2947. PubMed
The dendritic and epithelial cell receptor with a m.w. of 205 kDa (DEC205) is expressed by dendritic cells (DCs) and facilitates Ag presentation. After injection of Ags coupled to Abs specific for DEC205 into mice, Ag presentation occurs by nonactivated DCs, which leads to induction of regulatory T cells (Tregs). To test this system for tolerance induction in experimental allergic encephalomyelitis (EAE), we created single-chain fragment variables (scFv) specific for DEC205 and fused the scFv to the self-Ag myelin oligodendrocyte glycoprotein (MOG; scFv DEC:MOG). An anti-beta-galactosidase scFv:MOG fusion protein (scFv GL117:MOG) served as isotype control. After staining of DCs in vitro with purified scFv DEC:MOG, binding to DCs and colocalization with MHC class II was apparent, whereas isotype controls did not bind. We next injected scFv DEC:MOG into mice and observed elevated numbers of highly activated, IL-10-producing CD4(+)CD25(+)Foxp3(+) Tregs (17% of CD4) in spleens, as compared with isotype controls and uninjected mice (12% of CD4). Furthermore, DCs isolated from scFv DEC:MOG-injected animals produced significantly increased levels of TGF-beta. Most importantly, when EAE was induced in scFv DEC:MOG-injected mice, 90% of the mice were protected from EAE, whereas all mice in the isotype controls (scFv GL117:MOG) experienced development of EAE. When applying scFv DEC:MOG to mice that had already experienced EAE symptoms, abrogation of the disease in 90% of the animals was apparent, whereas all animals in the control groups experienced development of severe EAE. Thus, these data indicate that targeting of MOG to “steady-state” DCs in vivo may provide a tool to prevent and to treat EAE by a DC/Treg-driven mechanism.
in vitro TGFβ neutralization
Tai, N., et al. (2013). "TLR9 deficiency promotes CD73 expression in T cells and diabetes protection in nonobese diabetic mice" J Immunol 191(6): 2926-2937. PubMed
TLR9-deficient (TLR9(-)/(-)) NOD mice develop a significantly reduced incidence of diabetes. This study was to investigate the molecular mechanisms of the protective role of TLR9 deficiency. Through gene screening and confirmation by both mRNA and protein expression, we found a significant increase in CD73-expressing immune cells from peripheral lymphoid tissues in TLR9(-)/(-) NOD mice. The elevated frequency of CD73-expressing immune cells seemed to be specific for TLR9 deficiency and was MyD88 independent. Moreover, the increased frequency of CD73 expression was limited to the NOD background. Increased frequency of CD73 expression was also associated with lower levels of proinflammatory cytokines and more anti-inflammatory cytokine production in CD4(+) T cells in TLR9(-)/(-) NOD mice. Purified CD73(+)CD4(+) T cells showed stronger immunosuppressive function in vitro and delayed diabetes development in vivo. The immunosuppression appeared to be mediated by TGF-beta. In addition, elevated frequency of CD73-expressing cells was associated with improved beta cell function. Our observations were further confirmed by protection from diabetes with similar alterations in CD73 in the NY8.3 TCR NOD mouse model crossed with TLR9(-)/(-) mice and by the use of a TLR9 inhibitor in NOD mice. Our novel findings suggest an important immune-regulatory role of CD73 in regulation of diabetes development and may offer a new therapeutic strategy for specific intervention to prevent type 1 diabetes.
in vivo TGFβ neutralization
Kurkjian, C., et al. (2012). "Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection" Infect Immun 80(8): 2835-2846. PubMed
Pneumocystis pneumonia was first diagnosed in malnourished children and has more recently been found in children with upper respiratory symptoms. We previously reported that there is a significant delay in the immune response in newborn mice infected with Pneumocystis compared to adults (Garvy BA, Harmsen AG, Infect. Immun. 64:3987-3992, 1996, and Garvy BA, Qureshi M, J. Immunol. 165:6480-6486, 2000). This delay is characterized by the failure of neonatal lungs to upregulate proinflammatory cytokines and attract T cells into the alveoli. Here, we report that regardless of the age at which we infected the mice, they failed to mount an inflammatory response in the alveolar spaces until they were 21 days of age or older. Anti-inflammatory cytokines had some role in dampening inflammation, since interleukin-10 (IL-10)-deficient pups cleared Pneumocystis faster than wild-type pups and the neutralization of transforming growth factor beta (TGF-beta) with specific antibody enhanced T cell migration into the lungs at later time points. However, the clearance kinetics were similar to those of control pups, suggesting that there is an intrinsic deficiency in the ability of innate immunity to control Pneumocystis. We found, using an adoptive transfer strategy, that the lung environment contributes to association of Pneumocystis organisms with alveolar macrophages, implying no intrinsic deficiency in the binding of Pneumocystis by neonatal macrophages. Using both in vivo and in vitro assays, we found that Pneumocystis organisms were less able to stimulate translocation of NF-kappaB to the nucleus of alveolar macrophages from neonatal mice. These data indicate that there is an early unresponsiveness of neonatal alveolar macrophages to Pneumocystis infection that is both intrinsic and related to the immunosuppressive environment found in neonatal lungs.
in vivo TGFβ neutralization
Garidou, L., et al. (2012). "Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection" J Virol 86(13): 7060-7071. PubMed
Persistent viral infections often overburden the immune system and are a major cause of disease in humans. During many persistent infections, antiviral T cells are maintained in a state of immune exhaustion characterized by diminished effector and helper functions. In mammalian systems, an extensive immune regulatory network exists to limit unwanted, potentially fatal immunopathology by inducing T cell exhaustion. However, this regulatory network at times overprotects the host and fosters viral persistence by severely dampening adaptive immune responsiveness. Importantly, recent studies have shown that T cell exhaustion is mediated in part by host immunoregulatory pathways (e.g., programmed death 1 [PD-1], interleukin 10 [IL-10]) and that therapeutic blockade of these pathways either before or during persistent infection can promote viral clearance. Transforming growth factor beta (TGF-beta) is another immunosuppressive cytokine known to impede both self- and tumor-specific T cells, but its role in regulating antiviral immunity is not entirely understood. In this study, we inhibited TGF-beta with three potent antagonists to determine whether neutralization of this regulatory molecule is a viable approach to control a persistent viral infection. Our results revealed that these inhibitors modestly elevate the number of antiviral T cells following infection with a persistent variant of lymphocytic choriomeningitis virus (LCMV) but have no impact on viral clearance. These data suggest that therapeutic neutralization of TGF-beta is not an efficacious means to promote clearance of a persistent viral infection.